
A new study led by biological sciences professor Robert Hill and PhD student Xhoela Bame could have important implications for treating neurodegenerative diseases.
Generating new cells takes a lot of energy, so it might not come as a surprise that mitochondria, the "powerhouses" of the cell, are involved.
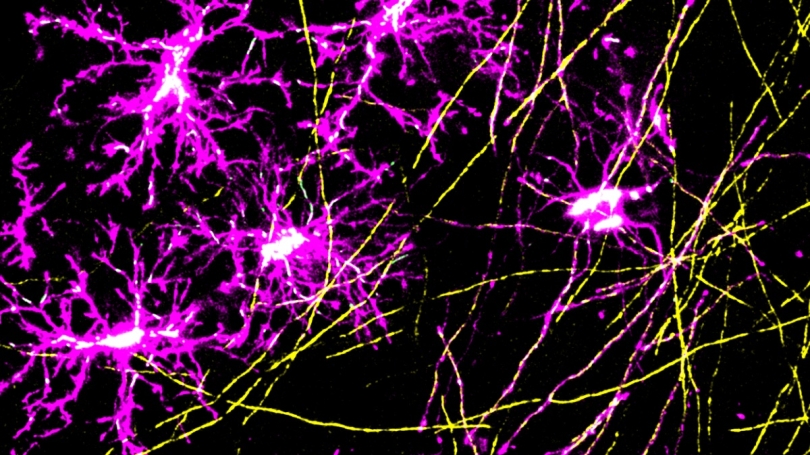
In a new study published in Nature Communications, Dartmouth scientists show that oligodendrocytes—supportive brain cells that are generated throughout life—undergo dramatic changes in the shape, location, and number of mitochondria that they harbor during their development. However, these dynamic mitochondrial changes are altered with age.
Understanding the molecular mechanisms behind oligodendrocyte generation could have important implications for aging and neurodegenerative diseases, since the flawless production of these cells is essential for brain function and repair.
"If we understand the process of how new oligodendrocyte cells are generated, we can extrapolate that to examine how they would regenerate if there was some defect, for example due to a disease or aging," says senior author Robert Hill, an assistant professor of biological sciences. "There's a drastic transition in mitochondria organization that takes place within just a few days."
Oligodendrocytes are long-lived cells within the central nervous system (brain and spinal cord) that provide a supportive role to the brain's neurons by wrapping them in a fatty, insulative material called myelin that speeds up the transmission of electrical impulses. Oligodendrocytes arise when oligodendrocyte precursor cells (or OPCs) undergo a process of maturation that takes around three days.
"A unique feature of these cells is that they can be generated and regenerated throughout life—it's a process that doesn't stop at development, but continues throughout adulthood," says Xhoela Bame, who led the project as a PhD candidate in Hill's research group. "We wanted to know how the intracellular environment influences this process to determine the 'fate' of the cells."
Specifically, Hill and Bame focused on the role of mitochondria, a type of organelle that is responsible for producing most of a cell's energy, since cellular development and myelin production are both energy-intensive processes.
To investigate the mitochondria's role in oligodendrocyte generation, the team used a microscopy technique that enabled them to observe the same OPCs and mitochondria at different timepoints over the course of several weeks. They used transgenic mice that had fluorescent markers attached to their OPCs and mitochondria and observed the cells while the mice were both awake and sedated.
"The power of this approach is that you can go back to the same part of a cell, the same mitochondria, and see how it changes in the intact system of the brain," Hill says.

They saw that as an OPC begins transitioning into an oligodendrocyte, its mitochondria migrate towards the cell's appendages and become elongated. During this stage, the cell is "spidery", with many appendages protruding from its central body that it uses to test out different neurons in the vicinity. Once it has selected a neuron to ensheath, it begins rapidly producing myelin to wrap around the neuron's axon. When this energy-intensive process is complete and the oligodendrocyte has reached maturity, its mitochondria migrate back to the central body or "soma" of the cell, and take on a shorter, rounder shape, become less mobile, and decrease in number.
When the researchers examined the mitochondria in human OPCs and oligodendrocytes from preserved brain samples, they observed similar differences: OPCs had more mitochondria than mature oligodendrocytes, and these mitochondria were localized to the cells' appendages, whereas oligodendrocyte mitochondria were more centrally located. The researchers say that these differences in mitochondrial features could be used to differentiate between old and newly developed oligodendrocytes.
"Mitochondria provide a lot of information about cell health and age, and, potentially, the capacity to generate a new mature oligodendrocyte," says Bame. "Just like you can count the rings on tree trunks to estimate their age, we can estimate the age of newly formed oligodendrocytes by looking at the cell's mitochondrial content, shape, and location."
Since oligodendrocyte generation can become impaired during aging, the team also compared mitochondria in the OPCs of young and elderly (18-21 months old) mice. They showed that in older mice, mitochondria were less mobile and shorter.
"We knew that OPCs can spontaneously generate new oligodendrocytes in the adult brain, but there was kind of a black box in terms of what's happening to all the organelles within that cell during that transition from precursor cell to mature oligodendrocyte," says Hill. "If we can understand the sub-cellular processes that occur during that transition, we might be able to manipulate those processes to help treat neurodegenerative conditions and aging."